Deep Expertise and Proven Success
REG SOLUTIONS was founded in 2014 as a dedicated Regulatory Consultant, focusing on Product Registration with the Saudi Food and Drug Authority (SFDA) and facilitating local marketplace international manufacturers of Medical Devices and Pharmaceuticals.
Our team possesses extensive knowledge of SFDA regulations and excels in navigating the complexities of the registration process. We are committed to delivering optimal compliance and tailored market access solutions, ensuring a smooth pathway for stakeholders to successfully enter the Saudi market.
At REG SOLUTIONS, we approach every task with the highest levels of professionalism, integrity, accuracy, and transparency. Our focus is on building long-term partnerships and providing strategic guidance to help you achieve your regulatory goals.
210+
7800+
Approved registrations
Happy clients

Our Services in Saudi Arabia


Product Classification System (PCS)
This service offers companies the opportunity to classify their Products and determine the level of Registration required, aligning with the Saudi FDA standards.




SFDA Medical Device Registration In Saudi Arabia
Our services include comprehensive registration of medical devices with the Saudi FDA in Saudi Arabia, supporting Establishment and Importation Authorization, as well as thorough analysis of applicable regulations.
Medical Device AR Licensing
Medical device manufacturers are required to designate an authorized representative (AR) in Saudi Arabia prior to launching their products in the market. ARs are accountable for ensuring both pre- and post-marketing regulatory compliance.






Medical Device Marketing Authorization (MDMA)
Medical Device Marketing Authorization (MDMA) is the official approval issued by the SFDA that permits companies to market and distribute medical devices and In-Vitro Diagnostics (IVDs) within Saudi Arabia.
Medical Device Importation & Distribution License
Looking to import medical devices into Saudi Arabia? Obtain your Medical Device Importation License quickly and efficiently with REG SOLUTIONS
National Center for Medical Devices Reporting (NCMDR)
The SFDA's (NCMDR) collects and evaluates data regarding the safety and performance of medical devices. This initiative enables stakeholders to report incidents, thereby enhancing safety and compliance in Saudi Arabia. Stakeholders can contact the Authorized Representative on behalf of the manufacturer for support in this process.

Request a Free Consultation
Schedule a free consultation with our regulatory specialists and discuss your specific needs. We'll tailor a solution to ensure your smooth and successful market entry in Kingdom of Saudi Arabia.
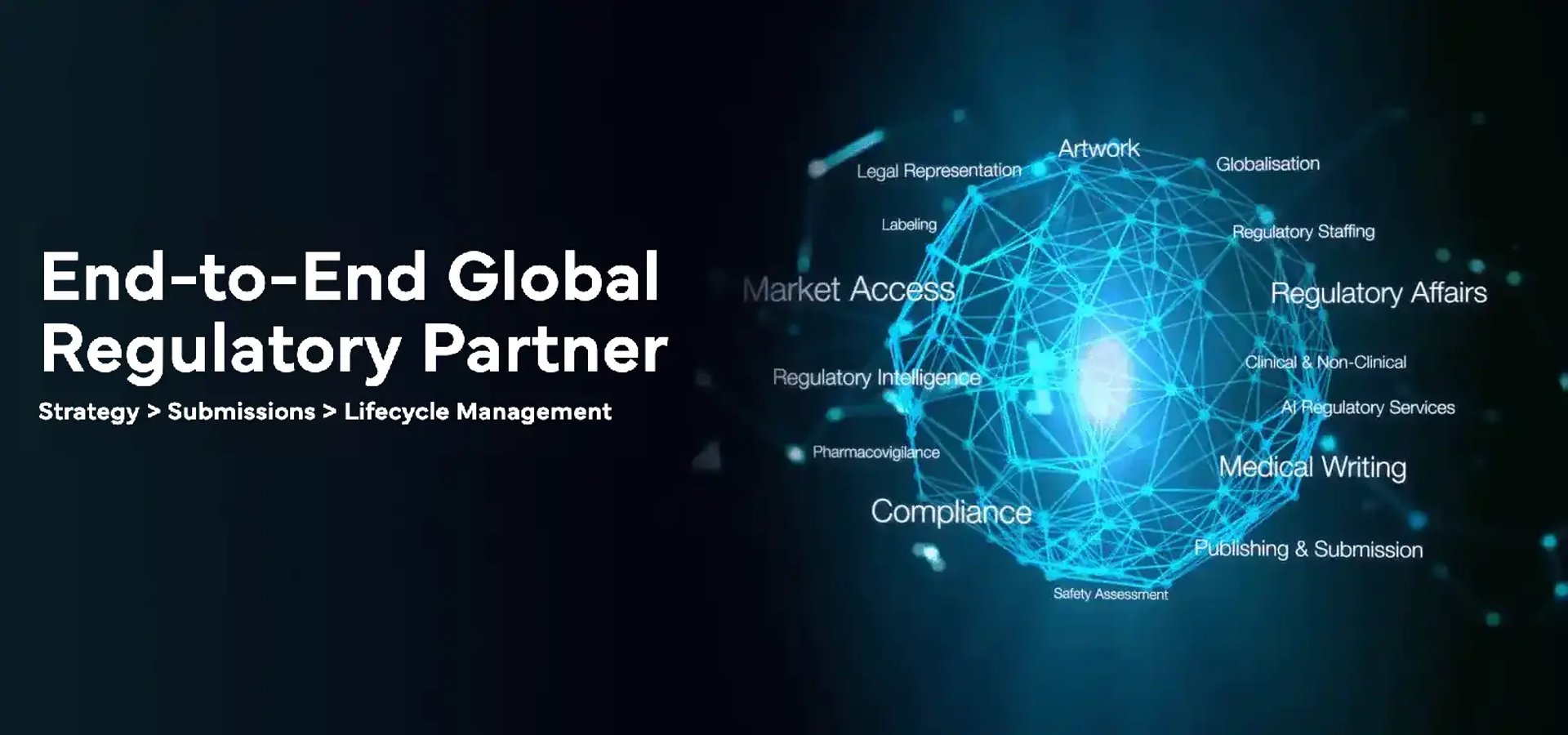
One of the Largest, Global, Regulatory-focused Solutions and Services Companies
Supporting, Large, Mid & Small global Life Sciences companies, (Pharmaceutical | Generics | Medical Device | Consumer Healthcare | Cosmetics | Biotechnology | Chemicals | Biosimilar | Biocides) in their entire Regulatory value-chain; ranging from Regulatory Strategy, Intelligence, Dossiers, Submissions etc. to Post- Approval / Legacy Product Maintenance, Labeling, Artwork Change Management and other related functions
Our medical regulatory compliance software offers innovative solutions that allow you to any market in the world ahead of your competitors
Management Systems Certification & Auditing
ISO Compliance Support
ISO 9001 ISO 13485 ISO 14001 ISO 15189 ISO 22716 and more
Coverage of over 100 markets and management assistance for product approvals in each and every one
Constant monitoring of global regulatory changes
We can help you expand to any market in the world.
Instant access to accurate and competitive regulatory intelligence
Utilization of artificial intelligence to deliver the most valuable and updated regulatory insights









Use of crowdsourcing to find answers to the most pressing regulatory questions

Reduce time to market and risk levels to provide advantages over competitors
Medical device and pharmaceutical companies spend months gathering intelligence from disparate sources and preparing product registration submissions, oftentimes only to be rejected by health authorities.
RegSolutions as medical regulatory company solves that problem.
The medical device industry is complex. You need regulatory compliance software that captures the unique needs of your business.
RegSolutions software is tailored to the medical device industry. Regulations are constantly evolving and that’s why our solution is continuously being monitored and improved.
With specialized regulatory compliance software, your business is only presented with the relevant information needed to make informed decisions. No more sorting through mountains of irrelevant data or conducting hours of research to move the process along or relying on distributors.
OUR ADVANTAGES


Client Testimonials
It has been an absolute pleasure working with RegSolutions over the past few months. Thank you very much for all the hard work – I could not imagine how difficult it would have been without the team being so proactive and diligent.
I look forward to returning to work with you in January and an exciting new chapter in our time.
– GRA Operations Program Manager, Top 5 Global Pharmaceutical Company
Thanks so much for all the help and diligence. We are so impressed by the way things have been handled by Fryer and the level of competence.
We have another very similar project coming up in the next few weeks where we need exactly the same level and type of service which we will be delighted to talk to you further.
– Program Manager, Regulatory Affairs – Global $1Bn Pharma Company
We would like to congratulate RegSolutions team for their excellent service and error free XEVPRM submission with short notice and well within the regulatory time frame. We sincerely appreciate their technical skills and their extraordinary and selfless effort.
We are pleased to acknowledge the timely support and recommend RegSolutions to everyone who are looking for such services.
– Vice President, Clinical Research & Pharmacovigilance, Microlabs